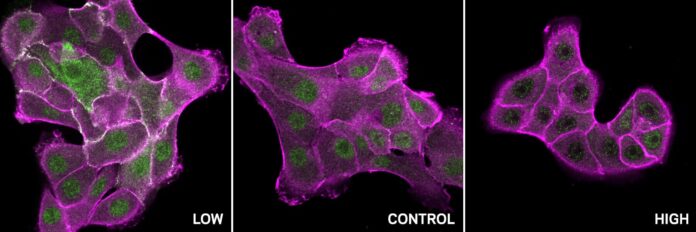
Tiny changes in acidity within our cells can have enormous impacts on their behavior. This influence comes from something called proton concentration, or pH – a measure of how acidic or alkaline a solution is. Proteins, the workhorses of our cells, are particularly sensitive to these shifts. Alterations in pH responsiveness can contribute to the development of diseases like cancer and neurodegenerative disorders like Alzheimer’s and Huntington’s.
For years, researchers have struggled to pinpoint exactly how proteins respond to changing pH levels. Traditionally, identifying pH-sensitive proteins involved a tedious process: meticulously testing each protein individually within a signaling pathway to see if it reacted to pH changes. This painstaking approach has yielded limited results. While scientists know pH plays a role in crucial cellular processes like cell movement and division, only around 70 cytoplasmic proteins have been confirmed as pH-sensitive out of countless possibilities.
Now, researchers at the University of Notre Dame have developed a groundbreaking computational tool that dramatically speeds up this process. This pipeline can analyze hundreds of proteins in just a few days, efficiently predicting which ones are sensitive to pH changes and potentially driving disease progression.
Unraveling the Molecular Code of pH Sensitivity
The new method relies on existing structural data of proteins housed in a global repository called the RCSB Protein Data Bank. The program then incorporates experimental data known as pKa values, which indicate the pH at which specific amino acids (the building blocks of proteins) gain or lose protons. This information allows the program to model how electrical charges within proteins shift depending on pH levels and predicts how these charge changes might alter protein structure.
The researchers focused their attention on amino acids that readily flip their charge within the narrow pH range typical of healthy cells (around 7.2 to 7.6). Even subtle shifts in these crucial amino acid charges can trigger a domino effect, cascading across the entire protein structure and impacting its function. This phenomenon is known as allostery – an indirect regulatory mechanism that influences protein activity far from the site where pH changes occur.
Targeting Cancer Through Computational Discovery
The pipeline successfully predicted the pH sensitivity of a key protein called SHP2 involved in cell signaling, immune response, and development. Experiments confirmed that binding protons to two specific residues within SHP2 causes its shape to change dramatically, switching it from an inactive “closed” state to an active “open” state. This discovery sheds light on how pH subtly controls SHP2 activity.
Furthermore, the pipeline identified a pattern of pH-sensitive sites in a wide range of SH2 domain-containing proteins, including c-Src. C-Src is often overactive in many cancers, driving uncontrolled cell growth. The analysis revealed that normal c-Src behaves differently at varying pH levels: it’s active at low pH and inactive at high pH. However, cancer-related mutations in these specific pH-sensitive sites disrupt this delicate regulation, rendering c-Src insensitive to pH changes and contributing to its overactivity in tumors.
This discovery opens exciting avenues for targeted cancer therapies. By understanding precisely how pH regulates c-Src activity, researchers can now design drugs that mimic the natural regulatory mechanisms, restoring normal pH sensitivity and selectively inhibiting the mutated, disease-driving form of the protein.
This groundbreaking computational tool promises to revolutionize our understanding of protein function in health and disease. Beyond cancer, targeting pH-sensitive proteins holds immense potential for treating a vast array of conditions where pH imbalances contribute to dysfunction – from diabetes and autoimmune disorders to traumatic brain injury. As researchers harness this new power, the future looks brighter for developing highly targeted and effective therapies.